In experiments of unprecedented scale, investigators at Weill Cornell Medicine and the National Institutes of Health have revealed new aspects of the complex genetics behind Type 2 diabetes. Through these discoveries, and by providing a template for future studies, this research furthers efforts to better understand, and ultimately, treat this common metabolic disease.
Previous studies have generally examined the influence of individual genes. In research described Oct. 18 in Cell Metabolism, senior co-author Dr. Shuibing Chen, the Kilts Family Professor of Surgery and a member of the Hartman Institute for Therapeutic Organ Regeneration at Weill Cornell Medicine, working alongside senior co-author Dr. Francis Collins, a senior investigator at the Center for Precision Health Research within the National Human Genome Research Institute of the U.S. National Institutes of Health, took a more comprehensive approach. Together, they looked at the contribution of 20 genes in a single effort.
“It’s very difficult to believe all these diabetes-related genes act independently of each other,” Dr. Chen said. By using a combination of technologies, the team examined the effects of shutting each down. By comparing the consequences for cell behavior and genetics, she said, “we found some common themes.”
As with other types of diabetes, Type 2 diabetes occurs when sugar levels in the blood are too high. In Type 2 diabetes, this happens in part because specialized cells in the pancreas, known as β-cells, don’t produce enough insulin, a hormone that tells cells to take sugar out of the blood for use as an energy source. Over time, high levels of blood sugar damage tissues and cause other problems, such as heart and kidney disease. According to the United States Centers for Disease Control and Prevention, nearly 9 percent of adults in the United States have been diagnosed with Type 2 diabetes.
Both genetic and environmental factors, such as obesity and chronic stress, can increase risk for it. Yet evaluating the role of the genetic contributors alone is a massive project. So far, researchers have identified more than 290 locations within the genome where changes to DNA can raise the likelihood of developing the disease. Some of these locations fall within known genes, but most are found in regions that regulate the expression of nearby genes.
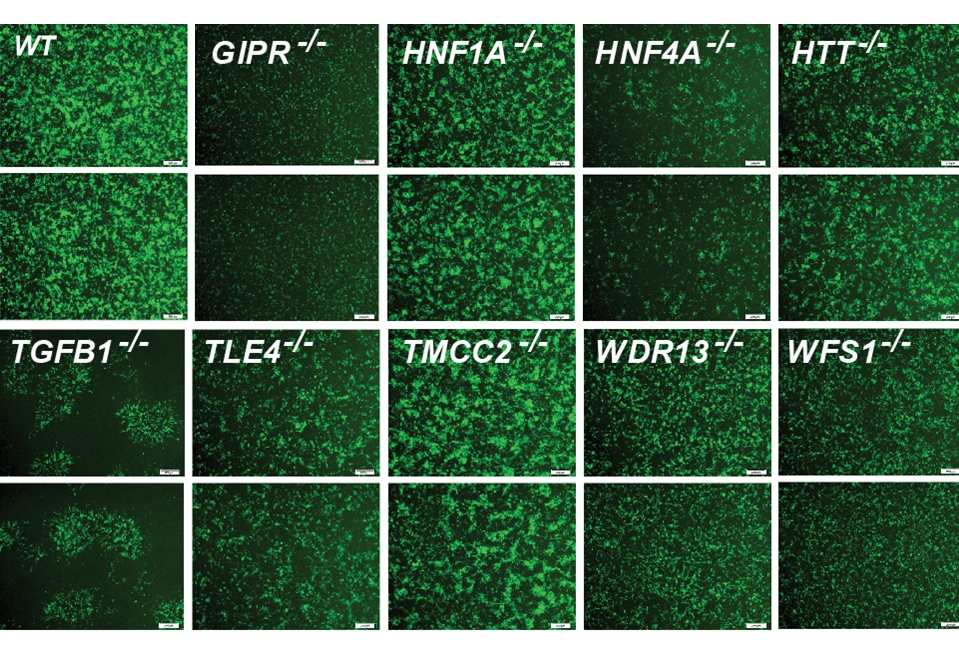
For the new research, the team focused on 20 genes clearly identified as contributors. They began their investigation by using the gene editing system CRISPR-Cas9 to shut down these genes, one at a time, within 20 sets of identical stem cells.
These stem cells had the potential to generate any kind of mature cell, but the researchers coaxed them into becoming insulin-producing β-cells. They then examined the effects of losing each gene on five traits related to insulin production and the health of β-cells. They also documented the accompanying changes in gene expression and the accessibility of DNA for expression.
To make sense of the massive amount of data they collected, the team developed their own computational models to analyze it, leading to several discoveries: By comparing the effects of all 20 mutations on β-cells, they identified four additional genes, each representing a newly discovered pathway that contributes to insulin production. They also found that, of the original 20 genes, only one, called HNF4A, contributed to all five traits, apparently by acting as a master controller that regulates the activity of other genes. In one specific example, they explained how a small variation, located in a space between genes, contributes to the risk of diabetes by interfering with HNF4A’s ability to regulate nearby genes.
Ultimately, this study and others like it hold the promise of benefiting patients, Dr. Collins said. “We need to understand all the genetic and environmental factors involved so we can do a better job of preventing diabetes, and to develop new ideas about how to effectively treat it.”
Drs. Collins and Chen note that their approach may have relevance beyond diabetes, to other common diseases, such as Alzheimer’s, Parkinson’s, and Crohn’s disease, that involve many genetic factors.
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, see the profile for Dr. Shuibing Chen.
The work reported in this newsroom story was supported in part by the United States’ National Institutes of Health grant 1-ZIA-HG000024, National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK124463, R01DK116075-01A1, R01DK119667-01A1, and 1U01DK127777-01 and an American Diabetes Association grant 9-22-PDFPM-06.

